2024
GENIUS™ Digital Diagnostics Letter
Dear Valued Customer,
As part of our ongoing commitment to providing innovation and excellence in cervical cancer screening, our laboratory is adopting Hologic’s newly FDA-cleared Genius™ Digital Diagnostics System to assist in AI-guided review of ThinPrep® Pap tests.
We will no longer offer the ThinPrep Imaging System for screening ThinPrep Pap tests.
The Genius Digital Diagnostics System with the Genius™ Cervical AI algorithm combines digital imaging and artificial intelligence. This assistive technology supplements human review to help identify pre-cancerous lesions and cervical cancer cells on ThinPrep Pap test cases.
Today, the patient’s cervical cells are collected and sent to the lab where they are transferred to a glass slide. To date, this glass slide has been reviewed under a microscope. With Genius Digital Diagnostics, the glass slides are digitally imaged, and an artificial intelligence algorithm is applied to curate a gallery of clinically relevant cells for cytologist and pathologist review.
The multi-site clinical study that supported FDA clearance found that review using Genius Digital Diagnostics increased HSIL+ sensitivity by 3.6% compared to the ThinPrep Imaging System.1
It also found that the detection of endocervical component increased by 6.2% compared to the ThinPrep Imaging System.1
Going forward, your ThinPrep Pap tests will be reviewed using the Genius Digital Diagnostics System. If you have questions, please reach out to a laboratory representative.
Considerations to aid in the transition to the Genius™ Digital Diagnostics System:
There is no change to specimen collection, as the sample is still collected and transferred into the ThinPrep Pap test vial.
There is no change to coverage for your patients; Genius Digital Diagnostics is covered by health plans in the same way asThinPrep Imaging.

Sincerely,
Carrie Chenault, MD
Medical Director
ProPath
For more information, please contact us at 214.638.2000.
2023
Oral & Maxillofacial Pathology Billing Focus
ProPath is excited to partner with dental and oral & maxillofacial clinicians to provide comprehensive pathology services to your patients. Led by Paras B. Patel, DDS, board-certified in Oral & Maxillofacial Pathology, ProPath can help provide solutions for your patient’s pathology needs.
Billing for Oral Pathology:
ProPath does NOT client bill. As a lab, billing for oral pathology services fall under a patient’s MEDICAL INSURANCE. Upon completing the order/requisition, please be sure the patient’s medical insurance information and demographics are sent to ProPath along with the specimen(s). This could be written on the requisition, copy of face sheet from PMS or copies of driver’s license and Medical insurance card (front and back). That’s it!
ProPath will take it from there! Patients of Medicare Age:
For patients who have Medicare Parts A & B, ProPath must bill Medicare Part B for the pathology services.
IMPORTANT: The referring clinician must be enrolled in Medicare’s PECOS (Provider Enrollment Chain & Ownership System) as an Ordering/Referring Provider. It is a database where physicians register with the Centers for Medicare and Medicare Services (CMS). CMS developed PECOS as a result of the Patient Protection and Affordable Care Act. If you are not enrolled, Medicare will deny the claim and ProPath may bill the practice for the services.
To learn more about this and enroll as an Ordering Provider, go to
Cost of Oral Pathology:
Most oral pathology cases are a biopsy charge (typically 88305). Sometimes, the pathologist may need to order a Decalcification (88311) or Special Stains (88312/88313) or Immunohistochemistry Stains (88342/88341). If Immunoflourescent Studies are ordered, there are always five (5) antibodies tested; 88346 x1 and 88350 x4. The additional testing completely depends on the pathologist and what is needed to render a diagnosis. Therefore, it is hard to predict the total cost of these services.
Most medical insurances will cover these services. Patients will only be responsible for any amounts applied to their deductible or coinsurance per their benefit plan. Average out-of-pocket for a single biopsy is approximately $50 and range can be from $5 - $110 depending on the insurance (based on current utilization).
Discount Programs:
ProPath offers several programs to assist patients with high deductibles or if they have no insurance:
- Uninsured Pricing – if a patient is uninsured, ProPath offers discounted pricing for all pathology services. The referring clinician must complete the “Certification of Uninsured Status” form (UNS) and submit with the requisition/order. ProPath also offers an annual Certification agreement and will supply stickers to the office to place on the requisition versus having to complete a form each time. Either way, patient will then receive this pricing up front. No further discounts will apply. For UNS Pricing information, please contact ProPath as indicated below.
- Payment Plans – if a patient owes a large balance, ProPath offers $0 interest payment plans which can be spread out on monthly payments up to 12 months, depending on the balance. Patients should contact our Billing Call Center at 800-654-1888 and a representative will be happy to assist.
- Financial Assistance – if a patient is medically/financially indigent, they can qualify for ProPath’s Financial Assistance Program. Applications can be obtained on ProPath website (www.propath.com). All applications require specific documentation for proof of household income, current medical debt etc. Patients should follow the instructions on the form.
For more information or questions related to billing for Oral Pathology services, contact:
ProPath Client Billing
Sarai Mendoza, at 214-237-1665 / [email protected] -OR- ProPath Oral Pathology Representative
Andrea Williams, at 817-542-5010 / [email protected]
For ProPath Billing questions, call 214-631-6721 / 800-654-1888 M-F, 7:30am-5:30pm CST
Reference Range
Dear Colleague,
As previously communicated, we recently changed chemistry and immunology analyzers. After doing so, it is common practice to re-evaluate reference ranges using acquired normal patient data. We have just completed a normal patient evaluation and have updated the reference ranges on the following chemistry analytes:
| Old | New | ||
| Alkaline Phosphatase | Adult Female | 35-104 U/L | 35-137 U/L |
| Calcium | Adult Range | 8.6 – 10.0 mg/dL | 8.6 – 10.5 mg/dL |
| CO2 | Normal Range | 20 – 29 mmol/L | 20 – 31 mmol/L |
| Creatinine | Adult Female | 0.51-0.95 mg/dL | 0.49-1.06 mg/dL |
| Adult Male | 0.7-1.3 mg/dL | 0.66-1.41 mg/dL | |
| *Fasting Glucose | Normal Range | <100 mg/dL | 70-99 mg/dL |
*We have updated the normal fasting glucose reference range based on CDC recommendations.
As always, our goal is transparency and good communication in our interactions with you so together we can provide the best patient care possible. If you have any concerns or questions, please feel free to contact me at 214.237.1670.
Thank you,
Carrie Chenault, MD
Medical Director, ProPath
Alpha-Fetoprotein, Maternal Serum (AFP)
Dear valued client,
Great news! To offer you and your patients a quicker turnaround time as well as quality results to which you have become accustomed, ProPath is now performing Alpha-Fetoprotein, Maternal Serum (AFP) Screening in-house. As you know, patient demographic information and especially an accurate estimated gestational age are essential to obtaining the best risk assessment analysis, therefore, we have put together this short guide to aid you in obtaining the required information.
ProPath’s AFP testing is validated for patients from
14 weeks - 24 weeks 6 days gestation.
For best results, please answer all ask at order entry (AOE) questions on your lab order form utilizing the outline below:
1. Gestation age (GA) as listed on sonogram: Answer: (Weeks.Days) (do not correct to current gestational age)
Answer: (Weeks.Days)
- First trimester sonogram is the most accurate measure of gestation age.
- Please provide the GA at SONOGRAM; not at time of collection unless same.
- Answer as N/A if no sonogram or if using LMP only to determine EDD.
2. Date of sonogram:
Answer: (MMDDYYYY)
- Answer as N/A if no sonogram or if using LMP only to determine EDD.
3. Date of LMP (last menstrual period):
Answer: (MMDDYYYY)
4. EDD (Estimated date of delivery):
Answer: (MMDDYYYY)
- If EDD determined by LMP; you may check your answer using the following link: http://www.medcalc.com/pregnancy.html
5. Maternal weight:
Answer: (lbs.ounces)
6. Insulin dependent:
Answer: (Y/N/U)
7. Number of gestations (fetuses):
Answer: (# fetus)
8. Race or ethnicity:
Answer: (Race/Ethnicity or NG [Not given])
9. Smoker:
Answer: (Y/N/U)
10. History of NTD:
Answer: (Y/N/U)
11. Initial sample/Repeat/Unknown:
Answer: (Initial/Repeat/Unknown)
If you have any questions, please contact Amy Doty, clinical specialist, at 214.490.2079.
Updated Collection Device and Testing Information
Please be aware of updated test code, test name, and collection device, M4 Viral Swab, required for SARS-CoV-2 (COVID-19), RT PCR testing. All updates/changes are in bold below.
Test Code/Test Name: SARS CoV-2/ SARS-CoV-2 (COVID-19), RT PCR
Specimen Container: M4 Viral Media and Swab
Collection requirements: Use swab from M4 Viral Swab packaging provided for any of these collection options: nasopharyngeal (collected by healthcare provider only); oropharyngeal (throat) (provider or self-collection); or nasal mid-turbinate collection (provider or self-collection)
**Please see back for detailed collection instructions for each option.
Once collection is complete, place tip of swab into viral transport media and snap off the applicator stick.
Transport Temperature: Critical Refrigerated
Specimen Stability: 72 hours refrigerated; 1 week frozen (-20 degrees)
Expected Turnaround Time: 4-7 days
Billing: Insurance or Uninsured patient (UNS form required)
CPT Code: 87635
M4 Viral Swab collection Kit
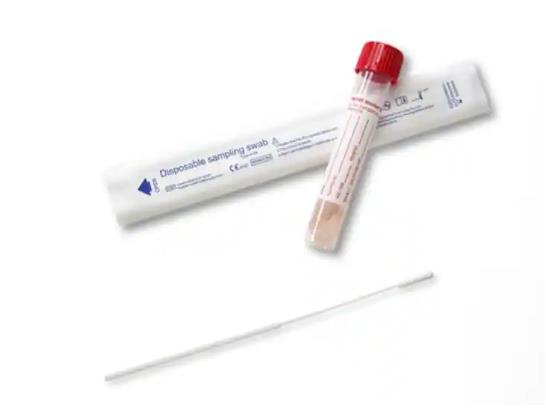
SARS-CoV-2 (COVID-19), RT PCR
Collection Instructions:
Nasopharyngeal: Tilt patient’s head back 70 degrees. Insert swab into nostril parallel to palate. (Swab should reach depth equal to distance from nostrils to outer opening of the ear.) Leave swab in place for several seconds to absorb secretions. Slowly remove swab while rotating it.
Nasal mid-turbinate: Tilt patient’s head back 70 degrees. While gently rotating the swab, insert swab less than one inch (about 2 cm) into nostril (until resistance is met at turbinates). Rotate the swab several times against nasal wall and repeat in other nostril using the same swab.
Oropharyngeal (throat): Insert swab into the posterior pharynx and tonsillar areas. Rub swab over both tonsillar pillars and posterior oropharynx and avoid touching the tongue, teeth, and gums.
Place tip of swab into viral transport media and snap off the applicator stick.
Core Lab Roche Switch
Dear Colleague,
We would like to thank you for continuing to entrust your patients to ProPath. Our goal is excellence in all aspects of diagnostic testing and patient care. With that in mind, we are updating our chemistry and immunology analyzers. These changes will better position us to provide for growing patient test volumes with continued excellence in quality and turnaround time. These new instruments come with new reference ranges so be sure to check the reference ranges on your reports. I would also like to highlight other important changes:
- The eGFR calculation for adults has been updated to the 2021 CKD-EPI creatinine equation.
- The new PSA is traceable to the Stanford Reference Standard/WHO 96/670. Results from this new method may not correlate with previous results from the old method, which was traceable to the Hybritech Standard.
- The anti-thyroglobulin antibody and anti-TPO antibody ranges and cutoff values have changed significantly, but the interpretations correlate well with the previous method.
- Thyroxine binding capacity (T-uptake) was indirectly measured on the old instrument but will be directly measured on the new instrument and reported as thyroxine binding index which, in turn, is used to calculate the free thyroxine index (fT4I).
- The total T3 units of measure have changed from ng/mL to ng/dL.
As always, our goal is transparency and good communication in our interactions with you so together we can provide the best patient care possible. If you have any concerns or questions, please feel free to contact me at 214.237.1670.
Thank you,
Carrie Chenault, MD
Medical Director, ProPath
Clinical Specimen Processing
In an ongoing effort to provide you and our shared patients with the best possible lab results, please share this communication with your laboratory specimen collection and processing staff.
Please follow these guidelines when processing blood specimens:
- Fill all blood tubes completely.
- After collection, mix all blood tubes with gentle inversion 4-5 times.
- Allow serum separator tubes (SST) to sit and clot for at least 30 minutes before placing in a centrifuge. Do not let serum tubes go unspun for more than 2 hours.
i. Utilize a secondary aliquot container when indicated, such as from red top tube collection or frozen serum.
ii. Record on any secondary aliquot container what has been aliquoted i.e serum, EDTA plasma, citrate plasma, etc. and 2 patient identifiers (full name, DOB) - For outside lockboxes
a. Include 1-2 cold packs (green zipper pouch) during the hot summer months. Do not place any blood specimens directly on the cold packs.
b. Place frozen specimens INSIDE blue zipper pouch with copy of the requisition.
For specimen collection requirements, stability, and turnaround time, please refer to the ProPath test menu at https://propath.com/for-healthcare-providers/test-menu/
Please contact your sales or service representative or Amy Doty, clinical specialist at 214-490-2789 with any questions.
We appreciate your assistance in yielding the best results for our patients.
Thank you.
Reference Ranges for Complete Blood Count (CBC) and Reticulocyte Count
We would like to thank you for continuing to entrust your patients to ProPath. Our goal is excellence in all aspects of diagnostic testing and patient care. With that in mind, we are notifying you that effective May 15, 2023, we have updated our hematology instrumentation and established new reference ranges for Complete Blood Count with and without Differential (CBC) and Reticulocyte (Retic) Count.
CBC with or without differential
| WBC | Male | Female | Units |
|
0 day - 15 days |
5.0 - 21.0 |
5.0 - 21.0 |
K/UL |
|
15 days - 30 days |
5.0 - 20.0 |
5.0 - 20.0 |
K/UL |
|
30 days - 60 days |
5.0 - 19.5 |
5.0 - 19.5 |
K/UL |
|
60 days - 6 months |
5.5 - 17.0 |
5.5 - 17.0 |
K/UL |
|
6 months - 2 years |
5.0 - 16.5 |
5.0 - 16.5 |
K/UL |
|
2 years - 6 years |
4.0 - 15.0 |
4.0 - 15.0 |
K/UL |
|
6 years - 8 years |
4.0 - 13.0 |
4.0 - 13.0 |
K/UL |
|
8 years - 10 years |
4.0 - 12.0 |
4.0 - 12.0 |
K/UL |
|
10 years - 12 years |
3.5 - 12.0 |
3.5 - 12.0 |
K/UL |
|
12 years - 18 years |
3.5 - 11.0 |
3.5 - 11.0 |
K/UL |
|
18 years - 150 years |
4.0 - 11.0 |
4.0 - 11.0 |
K/UL |
| RBC | Male | Female | Units |
|
0 day - 15 days |
3.90 - 6.30 |
3.90 - 6.30 |
M/UL |
|
15 days - 30 days |
3.60 - 6.20 |
3.60 - 6.20 |
M/UL |
|
30 days - 60 days |
3.00 - 5.40 |
3.00 - 5.40 |
M/UL |
|
60 days - 4 months |
2.70 - 4.90 |
2.70 - 4.90 |
M/UL |
|
4 months - 6 months |
3.10 – 4.50 |
3.10 - 4.50 |
M/UL |
|
6 months - 2 years |
3.70 - 5.30 |
3.70 - 5.30 |
M/UL |
|
2 years - 6 years |
3.90 - 5.30 |
3.90 - 5.30 |
M/UL |
|
6 years - 12 years |
4.00 - 5.30 |
4.00 - 5.30 |
M/UL |
|
12 years - 14 years |
4.30 - 5.80 |
4.00 - 5.40 |
M/UL |
|
14 years - 18 years |
4.50 - 6.10 |
4.00 - 5.40 |
M/UL |
|
18 years - 150 years |
4.60 - 6.10 |
4.60 - 6.10 |
M/UL |
| Hemoglobin | Male | Female | Units |
|
0 day - 15 days |
13.5 - 21.5 |
13.5 - 21.5 |
G/DL |
|
15 days - 30 days |
12.5 - 20.5 |
12.5 - 20.5 |
G/DL |
|
30 days - 60 days |
10.0 - 18.0 |
10.0 - 18.0 |
G/DL |
|
60 days - 4 months |
9.0 - 14.0 |
9.0 - 14.0 |
G/DL |
|
4 months - 6 months |
9.5 - 13.5 |
9.5 - 13.5 |
G/DL |
|
6 months - 2 years |
10.5 - 13.5 |
10.5 - 13.5 |
G/DL |
|
2 years - 6 years |
10.5 - 14.0 |
10.5 - 14.0 |
G/DL |
|
6 years - 8 years |
11.0 - 15.0 |
11.0 - 14.5 |
G/DL |
|
8 years - 10 years |
11.0 - 15.0 |
11.0 - 15.0 |
G/DL |
|
10 years - 12 years |
11.0 - 15.5 |
11.0 - 15.5 |
G/DL |
|
12 years - 16 years |
12.0 - 17.0 |
11.0 - 15.5 |
G/DL |
|
16 years - 18 years |
13.5 - 17.0 |
11.0 - 15.5 |
G/DL |
|
18 years - 150 years |
13.7 - 17.5 |
11.0 - 15.7 |
G/DL |
| Hematocrit | Male | Female | Units |
|
0 day - 15 days |
42.0 - 66.0 |
42.0 - 66.0 |
% |
|
15 days - 30 days |
39.0 - 63.0 |
39.0 - 63.0 |
% |
|
30 days - 60 days |
31.0 - 55.0 |
31.0 - 55.0 |
% |
|
60 days - 4 months |
28.0 - 42.0 |
28.0 - 42.0 |
% |
|
4 months - 6 months |
29.0 - 41.0 |
29.0 - 41.0 |
% |
|
6 months - 2 years |
30.5 - 42.0 |
30.5 - 42.0 |
% |
|
2 years - 6 years |
31.5 - 42.0 |
31.5 - 42.0 |
% |
|
6 years - 8 years |
33.0 - 43.0 |
33.0 - 43.0 |
% |
|
8 years - 10 years |
33.0 - 44.0 |
33.0 - 44.0 |
% |
|
10 years - 12 years |
33.0 - 45.0 |
33.0 - 45.0 |
% |
|
12 years - 14 years |
36.0 - 48.0 |
33.0 - 45.0 |
% |
|
14 years - 16 years |
36.0 - 50.0 |
33.0 - 45.0 |
% |
|
16 years - 18 years |
40.0 - 51.0 |
33.0 - 45.0 |
% |
|
18 years - 150 years |
40.0 - 51.0 |
34.0 - 45.0 |
% |
| MCV | Male | Female | Units |
|
0 day - 15 days |
88.0 - 26.0 |
88.0 - 126.0 |
fL |
|
15 days - 30 days |
86.0 - 24.0 |
86.0 - 124.0 |
fL |
|
30 days - 60 days |
85.0 - 23.0 |
85.0 - 123.0 |
fL |
|
60 days - 4 months |
77.0 - 15.0 |
77.0 - 115.0 |
fL |
|
4 months - 6 months |
74.0 - 08.0 |
74.0 - 108.0 |
fL |
|
6 months - 2 years |
70.0 - 86.0 |
70.0 - 86.0 |
fL |
|
2 years - 4 years |
70.0 - 87.0 |
70.0 - 87.0 |
fL |
|
4 years - 6 years |
72.0 - 87.0 |
72.0 - 87.0 |
fL |
|
6 years - 12 years |
75.0 - 90.0 |
75.0 - 90.0 |
fL |
|
12 years - 18 years |
78.0 - 95.0 |
78.0 - 95.0 |
fL |
|
18 years - 150 years |
79.0 - 99.0 |
79.0 - 97.0 |
fL |
| MCH | Male | Female | Units |
|
0 day - 60 days |
28.0 - 40.0 |
28.0 - 40.0 |
PG |
|
60 days - 4 months |
26.0 - 34.0 |
26.0 - 34.0 |
PG |
|
4 months - 6 months |
25.0 - 35.0 |
25.0 - 35.0 |
PG |
|
6 months - 6 years |
23.0 - 31.0 |
23.0 - 31.0 |
PG |
|
6 years - 12 years |
24.0 - 31.0 |
24.0 - 31.0 |
PG |
|
12 years - 16 years |
24.0 - 32.0 |
24.0 - 32.0 |
PG |
|
16 years - 18 years |
24.0 - 33.0 |
24.0 - 33.0 |
PG |
|
18 years - 150 years |
25.0 - 32.0 |
25.0 - 32.0 |
PG |
| MCHC | Male | Female | Units |
|
0 day - 6 months |
31.9 - 35.2 |
31.9 - 35.2 |
G/DL |
|
6 months - 2 years |
30.0 - 36.0 |
30.0 - 36.0 |
G/DL |
|
2 years - 6 years |
31.0 - 36.0 |
31.0 - 36.0 |
G/DL |
|
6 years - 12 years |
31.5 - 36.0 |
31.5 - 36.0 |
G/DL |
|
12 years - 18 years |
31.0 - 36.0 |
31.0 - 36.0 |
G/DL |
|
18 years - 150 years |
30.0 - 36.0 |
30.0 - 36.0 |
G/DL |
| RDW | Male | Female | Units |
|
All ages |
11.5 - 14.5 |
11.5 - 14.5 |
% |
| Platelet Count | Male | Female | Units |
|
0 day - 15 days |
200 - 450 |
200 - 450 |
K/UL |
|
15 days - 6 months |
200 - 600 |
200 - 600 |
K/UL |
|
6 months - 2 years |
200 - 750 |
200 - 750 |
K/UL |
|
2 years - 4 years |
200 - 600 |
200 - 600 |
K/UL |
|
4 years - 6 years |
200 - 550 |
200 - 550 |
K/UL |
|
6 years - 12 years |
200 - 500 |
200 - 500 |
K/UL |
|
12 years - 18 years |
150 - 450 |
150 - 450 |
K/UL |
|
18 years - 150 years |
160 - 340 |
170 - 450 |
K/UL |
| Absolute Neutrophils | Male | Female | Units |
|
0 day - 15 days |
1.50 - 0.00 |
1.50 - 10.00 |
K/UL |
|
15 days - 30 days |
1.00 - 9.50 |
1.00 - 9.50 |
K/UL |
|
30 days - 60 days |
1.00 - 9.00 |
1.00 - 9.00 |
K/UL |
|
60 days - 6 months |
1.00 - 8.50 |
1.00 - 8.50 |
K/UL |
|
6 months - 2 years |
1.50 - 8.50 |
1.50 - 8.50 |
K/UL |
|
2 years - 12 years |
1.50 - 8.00 |
1.50 - 8.00 |
K/UL |
|
12 years - 18 years |
1.50 - 7.50 |
1.50 - 7.50 |
K/UL |
|
18 years - 150 years |
1.70 – 5.40 |
1.50 - 6.10 |
K/UL |
| Absolute Lymphocytes | Male | Female | Units |
|
0 day - 30 days |
2.00 - 7.00 |
2.00 - 17.00 |
K/UL |
|
30 days - 60 days |
2.50 - 6.50 |
2.50 - 16.50 |
K/UL |
|
60 days - 6 months |
4.00 - 3.50 |
4.00 - 13.50 |
K/UL |
|
6 months - 2 years |
2.50 - 1.00 |
2.50 - 11.00 |
K/UL |
|
2 years - 4 years |
2.00 - 9.50 |
2.00 - 9.50 |
K/UL |
|
4 years - 6 years |
2.00 - 7.00 |
2.00 - 7.00 |
K/UL |
|
6 years - 8 years |
1.50 - 6.00 |
1.50 - 6.00 |
K/UL |
|
8 years - 14 years |
1.50 - 5.00 |
1.50 - 5.00 |
K/UL |
|
14 years - 16 years |
1.50 - 4.00 |
1.50 - 4.00 |
K/UL |
|
16 years - 18 years |
1.20 - 4.00 |
1.20 - 4.00 |
K/UL |
|
18 years - 150 years |
1.20 - 4.00 |
1.20 - 4.00 |
K/UL |
| Absolute Monocytes | Male | Female | Units |
|
0 day - 15 days |
0.30 - 3.60 |
0.30 - 3.60 |
K/UL |
|
15 days - 6 months |
0.30 - 2.70 |
0.30 - 2.70 |
K/UL |
|
6 months - 2 years |
0.10 - 1.40 |
0.10 - 1.40 |
K/UL |
|
2 years - 4 years |
0.10 - 1.30 |
0.10 - 1.30 |
K/UL |
|
4 years - 6 years |
0.10 - 1.10 |
0.10 - 1.10 |
K/UL |
|
6 years - 8 years |
0.10 - 1.00 |
0.10 - 1.00 |
K/UL |
|
8 years - 18 years |
0.10 - 0.90 |
0.10 - 0.90 |
K/UL |
|
18 years - 150 years |
0.20 - 1.00 |
0.20 - 1.00 |
K/UL |
| Absolute Eosinophils | Male | Female | Units |
|
0 day - 6 months |
0.00 - 1.10 |
0.00 - 1.10 |
K/UL |
|
6 months - 2 years |
0.00 - 0.90 |
0.00 - 0.90 |
K/UL |
|
2 years - 12 years |
0.00 - 0.70 |
0.00 - 0.70 |
K/UL |
|
12 years - 18 years |
0.00 - 0.50 |
0.00 - 0.50 |
K/UL |
|
18 years - 150 years |
0.00 - 0.50 |
0.00 - 0.50 |
K/UL |
| RDW | Male | Female | Units |
|
All ages |
0.0 - 0.08 |
0.0 - 0.08 |
K/UL |
Reticulocyte Count
Notes: Effective 5/15/23, reference ranges updated to the following:
| Reticulocyte Count | Male | Female | Units |
|
0 day - 4 days |
3.47 - 5.40 |
3.47 - 5.40 |
% |
|
4 days - 30 days |
1.06 - 2.37 |
1.06 - 2.37 |
% |
|
30 days - 60 days |
2.12 – 3.47 |
2.12 - 3.47 |
% |
|
60 days - 6 months |
1.55 - 2.70 |
1.55 - 2.70 |
% |
|
6 months - 2 years |
0.99 - 1.82 |
0.99 - 1.82 |
% |
|
2 years - 6 years |
0.82 - 1.45 |
0.82 - 1.45 |
% |
|
6 years - 12 years |
0.98 - 1.94 |
0.98 - 1.94 |
% |
|
12 years - 18 years |
0.90 - 1.49 |
0.90 - 1.49 |
% |
|
18 years - 150 years |
0.80 - 2.90 |
0.80 - 2.90 |
% |
| Absolute Reticulocyte | Male | Female | Units |
|
0 day - 4 days |
147.5 - 16.4 |
147.5 - 16.4 |
K/UL |
|
4 days - 30 days |
51.3 - 110.4 |
51.3 - 110.4 |
K/UL |
|
30 days - 60 days |
51.8 - 77.9 |
51.8 - 77.9 |
K/UL |
|
60 days - 6 months |
48.2 - 88.2 |
48.2 - 88.2 |
K/UL |
|
6 months - 2 years |
43.5 - 111.1 |
43.5 - 111.1 |
K/UL |
|
2 years - 6 years |
36.4 - 68.0 |
36.4 - 68.0 |
K/UL |
|
6 years - 12 years |
42.4 - 70.2 |
42.4 - 70.2 |
K/UL |
|
12 years - 18 years |
41.6 - 65.1 |
41.6 - 65.1 |
K/UL |
|
18 years - 150 years |
35.0 - 134.0 |
32.0 - 105.0 |
K/UL |
2023 Annual Notice to Physicians
Date: 05/19/2023
To: ProPath Client
From: Compliance Department
Re: 2023 Annual Notice to Physicians
ProPath is providing annual notification to our clients of the Medicare policies governing the ordering and reimbursement of laboratory tests. ProPath is committed to promoting awareness of and adherence to these policies. In accordance with the Office of the Inspector General’s (OIG) Compliance Program Guide for Clinical Laboratories, we are providing the following information about Medicare requirements:
CMS Medical Necessity Policy
Medicare will only pay for tests that meet the Medicare definition of “medical necessity”. Medicare may deny payment for a test that the physician believes is appropriate, such as a screening test, which does not meet the Medicare definition of medical necessity. All diagnosis and clinical relevance for patient treatment should be noted in the patient’s chart.
The OIG takes the position that an individual who knowingly causes a false claim to be submitted may be subject to sanctions or remedies available under civil, criminal and administrative law.
Individuals who knowingly cause a false claim to be submitted to Medicare may be subject to sanctions or remedies available under civil, criminal and administrative law.
CMS Signature Requirements
According to CMS’ guidance on laboratory services documentation requirements, unsigned requisitions alone do not support physician intent to order. Physicians should sign all orders for diagnostic services to avoid potential denials. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/LabServices-ICN909221-Text-Only.pdf
Medicare Laboratory National Coverage Determinations (NCDs) and Local Coverage Determinations (LCDs)
Coverage determination policies define medical conditions through the inclusion on a list of ICD (diagnosis) codes for which these tests are covered or reimbursed by Medicare. HIPAA regulations require ICD codes to be present on each claim filed. These codes must also be documented in the patient’s medical record.
NCDs: https://www.cms.gov/Medicare/Coverage/CoverageGenInfo/LabNCDsICD10.html
LCDs: https://www.novitas-solutions.com/webcenter/portal/NovitasSolutions
Novitas Solutions Medicare Jurisdiction H
Frequency Limitations for Laboratory Tests
Certain laboratory tests have specific frequency limitation requirements. The limitations may apply to tests that are included in NCDs and LCDs.
Medicare Preventive Screening Laboratory Tests
Certain preventive screening laboratory tests are covered services for Medicare beneficiaries. Benefit coverage is specific for each service, diagnosis codes, coverage requirements, and frequency limitations. https://www.cms.gov/Medicare/Prevention/PrevntionGenInfo/medicare-preventive-services/MPS-QuickReferenceChart-1.html
American Medical Association (AMA) Organ or Disease-Oriented Panels
The AMA panels were developed for coding purposes only and should not be interpreted as clinical parameters. Organ and disease-oriented panels will only be paid by Medicare when all tests within the panel are deemed medically necessary by Medicare.
https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c16.pdf Section 90.2 – Organ or Disease Oriented Panels
In the case of custom test panels, all individual tests must meet medical necessity guidelines.
Reflex Testing
Reflex testing occurs when initial test results indicate that a second related test is medically appropriate or required by state, regulatory, or accreditation standards. Most tests can be ordered without a reflex. Find details at https://www.sonichealthcareusa.com/ap/testing-solutions/test-menu/.
Advance Beneficiary Notice of Non-Coverage (ABN)
- Limited Coverage – An ABN is required if the diagnosis is not covered
- Frequency Limit - An ABN is required at each encounter for frequency limited tests
- Non-Coverage – An ABN is required for experimental or research use tests or tests designated by Medicare as non-covered
https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs.html
Manual 100-04 Medicare Claims Processing Manual
Chapter 30 Financial Liability Protections
Section 50 Form CMS-R-131 Advance Beneficiary Notice of Non-Coverage (ABN)
Medicare Clinical Laboratory Fee Schedule (CLFS)
Medicare reimbursement for laboratory CPT/HCPCS codes is located at.
Additional details can be found at PAMA regulations. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/PAMA-Regulations.html
Medicaid reimbursement amount will be equal to, or less than, the amount of Medicare reimbursement.
Medicare Part B National Correct Coding Initiative (NCCI) Edits
The Medicare NCCI was implemented to promote national correct coding methodologies and to control improper coding leading to inappropriate payment. https://www.cms.gov/Medicare/Coding/NationalCorrectCodInitEd/index.html
Contact Information
The Medical Directors and other pathologists are available to discuss appropriate testing and test ordering. Please call 214.638.2000 for assistance. You may also contact our Compliance Department at [email protected].
Please review this notice with all appropriate staff.
Thank you for supporting ProPath.

