By Kathleen M. Murphy, Ph.D
Background: Cetuximab (Erbitux®) and Panitumumab (Vectibix®) are monocolonal antibody therapies that target the Epidermal Growth Factor Receptor (EGFR). These agents are approved by the Food and Drug Administration (FDA) for use in patients with colorectal cancer that is metastatic or has failed to respond to other chemotherapeutic treatments. Less than 25% of patients treated with EGFR-targeted agents will respond and benefit from these therapies. It is therefore important to identify patients that will not benefit from these therapies in order to avoid potential side effects, and to avoid the significant costs associated with these treatments.
Clinical Significance: Recent data indicate that the presence of a codon 12, 13, or 61 KRAS gene mutation or a V600E mutation of the BRAF gene results in resistance to these anti-EGFR monoclonal antibody therapies. Patients with colorectal cancers harboring these mutations do not derive benefit from administration of EGFR-targeted monoclonal antibody therapy in the first-line, second-line, or third-line settings. Current guidelines from the National Comprehensive Cancer Network (NCCN) recommend the use of anti-EGFR monoclonal antibody therapies only in patients with wild type (negative for mutation) KRAS colorectal carcinoma.
The Assay Performed at ProPath®: At ProPath, we use pyrosequencing technology to detect mutations in codons 12, 13, and 61 of the KRAS gene and the V600E mutation of the BRAF gene. Our assay offers several advantages over other assays including:
• All tissues are reviewed by our expert pathologists to ensure adequacy for testing, and to identify the area of tissue most suitable for analysis.
• The assay detects mutations in 3 codons of KRAS (12, 13, and 61), while many other assays only evaluate codons 12, and 13.
Additionally, the assay detects essentially all mutations in these codons, rather than just the 7 most common mutations.
• Pyrosequencing has a better limit of detection (approximately 5%) compared to standard sanger sequencing (approximately 20%), which results in fewer false negatives due to low levels of tumor in the specimen.
• Clinicians BRAF have the flexibility to order KRAS and BRAF individually or as a panel.
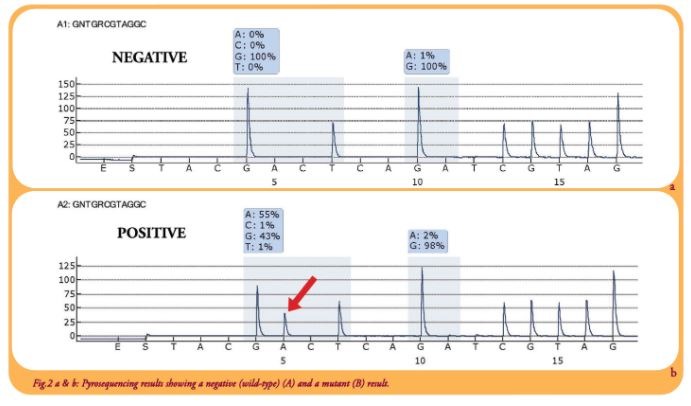
This assay is performed on formalin-fixed paraffin-embedded tumor specimens. Turnaround time is 3-6 days from the time the specimen arrives in the laboratory.
References:
1. NCCN Clinical Practice Guidelines in Oncology; Colon Cancer; v.4.2008
2. Jimeno A et al. The Cancer Journal. 2009 Vol 15, No 2. p110-113
3. Loupakis F et al. Br J Cancer. 2009 Aug 18;101(4):p715-721
