By Craig E. Litz, M.D.
Gastric intestinal (goblet cell) metaplasia (GIM), originally described in the 19th century, is defined as the presence of intestinal type cells in the gastric mucosa.1 This definition specifically excludes intestinal metaplasia of columnar epithelium in the esophagus (Barrett’s esophagus). Normally, the gastric mucosal epithelium is composed of 5 cell types: mucous neck and columnar cells which are located throughout the stomach; and parietal, chief, and endocrine cells which are found in the fundus and body (fig.1).
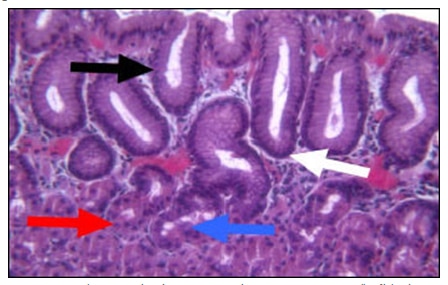
GIM is a relatively common abnormality that usually occurs in the setting of chronic inflammation and is distinguished by the presence of goblet cells, cells in which the cytoplasmic membrane and nucleus are distorted by large vacuoles of acidic (intestinal) mucin on hematoxylin and eosin stained sections (fig. 2A, black arrow).1 Goblet cells may be confirmed by Alcian Blue/PAS cytochemical stains. The Alcian Blue stains the acid mucin of goblet cells blue while the PAS stains all mucin red (fig. 2B, black and white arrows, respectively). GIM typically begins at the junction of the gastric antrum and corpus as foci that may appear endoscopically as small nodules. These foci eventually fuse and extend distally to involve the entire antrum and proximally to involve the entire lesser curvature. It may also extend in finger-like processes to the proximal oxyntic mucosa on the anterior and posterior walls of the gastric body.
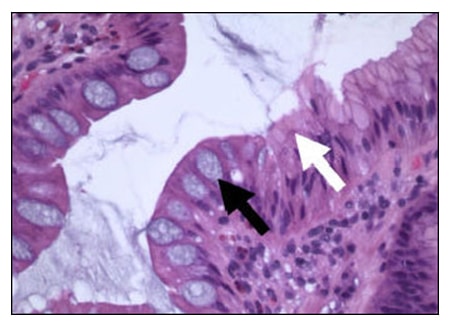
Fig. 2A
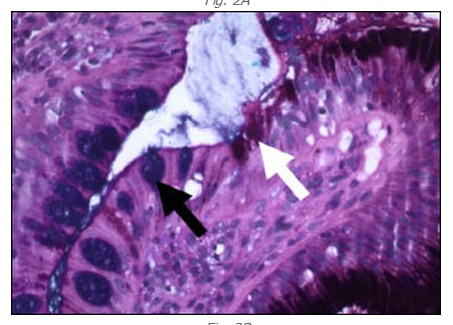
Fig. 2B
Fig. 2A and 2B – Gastric mucosa showing goblet cell (intestinal) metaplasia stained with H and E (A) and Alcian Blue/PAS (B). Goblet cells (black arrows) show clear cytoplasmic vacuole that displaces the nucleus on H and E (Panel A) and stains blue with Alcian Blue/PAS (Panel B). Gastric mucous cells are identified in both stain preparations by the white arrows. 100X.
In contrast, GIM associated with pernicious anemia is limited to the oxyntic mucosa and spares the antrum.2 Several investigators have proposed the concept of “complete” and “incomplete” gastric intestinal metaplasia.3,5 Completely metaplastic epithelium is composed of intestinal goblet and absorptive cells; incomplete metaplasia consists of a mixture of goblet cells and mucin-secreting gastric cells. Further subclassification has been proposed based on whether the mucin produced in the metaplastic epithelium is colonic (sulfomucin; type III) or small bowel (sialomucin) in nature and whether there is glandular distortion accompanying the metaplasia.5 Unfortunately, criteria for classifying intestinal metaplasia have been confusing and sometimes vague not allowing practical application. No consensus exists regarding the diagnostic reproducibility of the various subclasses of gastric intestinal metaplasia.1,6,7
As a result, the clinical significance of gastric intestinal metaplasia in biopsy material is debated. It is generally agreed that:
1) Intestinal metaplasia is a common finding in biopsy material. In one large study, 244 of 1041 (23%) endoscopic biopsy specimens contained intestinal metaplasia of one type or the other.5
2) The extent of intestinal metaplasia is related to the risk of carcinoma.8 However, it is difficult to judge the extent of gastric intestinal metaplasia based on small biopsy specimens. Serological tests for pepsinogen group I (PGI) and group II (PGII) have been useful in this regard. PGI is produced by gastric chief cells of the corpus while PGII is synthesized in the gastric antrum and cardia, and Brunner’s glands of the duodenum. As chief cells are replaced by metaplastic intestinal mucosa, PGI levels fall while PGII levels remain relatively static. When this happens, the PGI/PGII ratio falls.9 Miki et al showed there is a good correlation between the surface area of the acid-producing mucosa, the maximum acid output, and the PGI to PGII ratio.10
3) Currently, the distinction of the various subtypes of intestinal metaplasia is of no practical value in assigning carcinoma risk to patients. Some investigators have suggested that type III metaplasia is associated with intestinal gastric carcinoma but other studies have not confirmed this.1,5,11,12 The differing protocols used by the various studies in determining this type of metaplasia, the retrospective nature of most studies and the lack of prospective studies to rigorously evaluate the relationship of type III GIM to gastric carcinoma have not allowed any definite conclusions to be reached.
In summary, while patients with extensive GIM show an increased risk for gastric carcinoma, the value of subtyping GIM in assigning carcinoma risk in any given patient awaits better designed prospective studies with easily applied, reproducible criteria for pathologists.
References:
1. Stemmermann GN. Intestinal metaplasia of the stomach. A status report. Cancer. 1994;74:556-564
2. Stemmermann GN, Hayashi T. Intestinal metaplasia of the gastric mucosa: a gross and microscopic study of its distribution in various disease states. J Natl Cancer Inst. 1968;41:627-634
3. Matsukura N, Suzuki K, Kawachi T, Aoyagi M, Sugimura T, Kitaoka H, Numajiri H, Shirota A, Itabashi M, Hirota T. Distribution of marker enzymes and mucin in intestinal metaplasia in human stomach and relation to complete and incomplete types of intestinal metaplasia to minute gastric carcinomas. J Natl Cancer Inst. 1980;65:231-240
4. Segura DI, Montero C. Histochemical characterization of different types of intestinal metaplasia in gastric mucosa. Cancer. 1983;52:498-503
5. Silva S, Filipe MI. Intestinal metaplasia and its variants in the gastric mucosa of Portuguese subjects: a comparative analysis of biopsy and gastrectomy material. Hum Pathol. 1986;17:988-995
6. Antonioli DA. Precursors of gastric carcinoma: a critical review with a brief description of early (curable) gastric cancer. Hum Pathol. 1994;25:994-1005
7. Lauwers GY. Defining the pathologic diagnosis of metaplasia, atrophy, dysplasia, and gastric adenocarcinoma. J Clin Gastroenterol. 2003;36:S37-43; discussion S61-32
8. Kato I, Tominaga S, Ito Y, Kobayashi S, Yoshii Y, Matsuura A, Kameya A, Kano T, Ikari A. A prospective study of atrophic gastritis and stomach cancer risk. Jpn J Cancer Res. 1992;83:1137-1142
9. Stemmermann GN, Samloff IM, Nomura AM, Heilbrun LK. Serum pepsinogens I and II and stomach cancer. Clin Chim Acta. 1987;163:191-198
10. Miki K, Ichinose M, Shimizu A, Huang SC, Oka H, Furihata C, Matsushima T, Takahashi K. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol Jpn. 1987;22:133-141
11. Ectors N, Dixon MF. The prognostic value of sulphomucin positive intestinal metaplasia in the development of gastric cancer. Histopathology. 1986;10:1271-1277
12. Ramesar KC, Sanders DS, Hopwood D. Limited value of type III intestinal metaplasia in predicting risk of gastric carcinoma. J Clin Pathol. 1987;40:1287-1290
