By Ravi Patel, M.D.
A 52-year-old female presents with watery diarrhea of several weeks. Her past medical history is unremarkable. She was also due for a screening colonoscopy and so underwent a colonoscopy which showed normal mucosa. No polyps, erythema, or ulcer were seen. The endoscopist performed multiple random biopsies throughout the colon. On microscopy, epithelial denudation, intraepithelial lymphocytes and a thickened collagen table were identified. The diagnosis of collagenous colitis was rendered.
 Collagenous colitis (CC) is not an uncommon colon disorder and in our largely outpatient population practice has been observed regularly. A known cause of long standing unexplained watery diarrhea, CC is characterized by a chronic collagen depositing colitis without architectural distortion which on colonoscopy usually shows normal mucosa. Collagenous colitis was first reported in 1976 by Lindstrom after identifying a case of colorectal subepithelial collagen deposition with notable morphologic similarity to the previously described collagenous sprue (1). In 1947, an idiopathic sprue with eosinophilic hyaline deposits was initially observed (2). This eventually led to the recognition and formal coining of “collagenous sprue” in 1970 when similar hyaline deposits were confirmed to be collagen by electron microscopy (3,4). There is a female preponderance and this condition mostly affects middle-aged to older adults. In a recent systematic review with meta-analysis, Tong et al looked at 25 studies and pooled the data to have found an incidence rate of 4.14 per 100,000 person-years, female to male incidence ratio of 3.05, and 64.9 years for the median affected age (5). While non-bloody watery diarrhea is the most common manifestation, fecal incontinence, abdominal pain, and urgency may be present. Autoimmune diseases are often coexistent such as autoimmune thyroiditis, celiac sprue, or rheumatoid arthritis. While usually normal endoscopically, infrequently erythema, friability, or ulcerations can be seen.
Collagenous colitis (CC) is not an uncommon colon disorder and in our largely outpatient population practice has been observed regularly. A known cause of long standing unexplained watery diarrhea, CC is characterized by a chronic collagen depositing colitis without architectural distortion which on colonoscopy usually shows normal mucosa. Collagenous colitis was first reported in 1976 by Lindstrom after identifying a case of colorectal subepithelial collagen deposition with notable morphologic similarity to the previously described collagenous sprue (1). In 1947, an idiopathic sprue with eosinophilic hyaline deposits was initially observed (2). This eventually led to the recognition and formal coining of “collagenous sprue” in 1970 when similar hyaline deposits were confirmed to be collagen by electron microscopy (3,4). There is a female preponderance and this condition mostly affects middle-aged to older adults. In a recent systematic review with meta-analysis, Tong et al looked at 25 studies and pooled the data to have found an incidence rate of 4.14 per 100,000 person-years, female to male incidence ratio of 3.05, and 64.9 years for the median affected age (5). While non-bloody watery diarrhea is the most common manifestation, fecal incontinence, abdominal pain, and urgency may be present. Autoimmune diseases are often coexistent such as autoimmune thyroiditis, celiac sprue, or rheumatoid arthritis. While usually normal endoscopically, infrequently erythema, friability, or ulcerations can be seen.
There is a female preponderance and this condition mostly affects middle-aged to older adults.
The cause of CC is unknown; however, several theories have been proposed. Most of these supports the notion that luminal bacterial, drug, or dietary elements contain antigens which trigger localized immune response at the level of the gut mucosa. For instance, abnormal regulation of collagen metabolism can happen from metalloproteinase (TIMP-1) imbalance resulting in decreased collagen degradation (6). Epithelium interleukin level changes were seen to vary with ileostomy reversals in patients with CC, and luminal antigen stimulation was postulated to play a role when diversion of the fecal stream resulted in regression of CC histology (6). Drugs such as NSAIDs, PPIs, statins, and SSRIs have been linked to CC, and microscopic colitis in general.7 Hence, the prevailing belief is that a fecal antigen triggers a leaky membrane and leads to the dysregulation of cytokines, deposition of collagen, and retention of intestinal fluids resulting in watery diarrhea.
The histologic features for collagenous colitis can be patchy or diffuse. The hallmark finding is that of subepithelial collagen deposition that is 10 microns to 60 microns. Normal colons have a subepithelial basement membrane that is 2 to 5 microns. For reference, a typical RBC is 6-8 microns, a typical neutrophil is 10-16 microns. While the absolute thickness may be a useful parameter to keep in mind, the recognition of thickened membranes in terms of its size relative to adjacent leukocytes and surface epithelial columnar cells is more practical. The collagen collects just below the surface columnar epithelium and in doing so it entraps fine capillaries, degenerated hyperchromatic epithelial nuclei, and leukocytes. Moreover, the collagen table has a notable irregular lower border. Frequently, the surface is damaged with areas of epithelial denudation and superficial erosions overlying thickened collagen membranes. (See Figure 1). Increased intraepithelial lymphocytes are seen, although to a lesser degree than lymphocytic colitis. The intraepithelial lymphocytes are generally found in the surface epithelium and to a lesser degree in the crypts. Few intraepithelial eosinophils and sparse neutrophils can be seen along with occasional cryptitis. However, the presence of prominent neutrophils or crypt abscesses should not be seen. On rare occasions, multinucleate giant cells may be entrapped in the collagen table. Mild to moderate expansion of the lamina propria occurs with lymphocytes and plasma cells in a “top-heavy” distribution. There should be no crypt distortion, and any minimal crypt distortion is limited to an area of erosion. There should not be epithelial atrophy or hemorrhage.
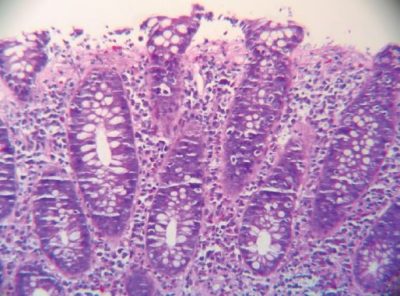
Fig.1: High Power H&E. Subepithelial collagen deposit with overlying epithelial denudation.
With a trichrome stain, not only is the shear thickness and subepithelial nature of the collagen evident, but also irregular process-like collagenous extensions are seen dipping into the underlying lamina propria. The trichrome stain shows a thickened band-like plate in blue against a deep red background of cells and white of mucin and loose connective tissue. The colonic crypt basement membrane is spared (See Figure 2).
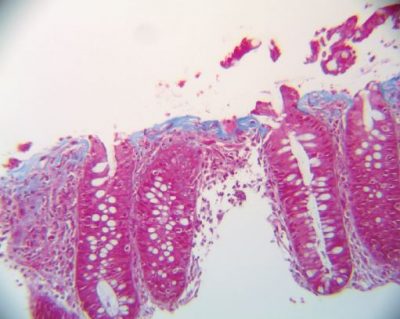
Fig.2: High power Trichrome. The irregular nature of the collagen is seen in blue.
To diagnose collagenous colitis accurately, a constellation of histologic features should be present, and CC should be rendered in the appropriate clinical context to avoid over or underdiagnosing this entity. In literature the buzzwords “collagen thickening” or “collagen deposition” are often misconstrued to be pathognomonic with CC; however, the presence of conspicuous collagen alone is not enough to diagnose collagenous colitis and should be avoided to prevent false positives. Nonspecific collagen deposition or hyalinization may be seen in radiation proctitis, ischemia or in mucosal prolapse. Small pieces of tissue often round up during formalin fixation and can result in tangential sections which appear to accentuate the subepithelial collagen and therefore tangential areas should be ignored in lieu for flat, well-oriented pieces of tissue. In practice, there should be a history of chronic abdominal symptoms, notably diarrhea, before rendering CC, unless the microscopic findings show definite evidence for CC. In such a case, the clinical finding may not have been reported on the endoscopy report or even by the patient to the clinician. For example, this may happen as an underreported finding in an otherwise normal patient receiving colonoscopy for cancer screening who simply forgot to report loose stools or other atypical symptoms. This could prompt a discussion between the pathologist and the gastroenterologist. In practice such cases may be signed out descriptively with a comment that raises this possibility. Furthermore, collagenous colitis is well-known to show patchy involvement of the colon meaning that certain parts of the colon would be histologically normal whereas other portions of the large bowel would show histologic pathology. To further add to the conundrum, the morphologic changes themselves often vary from one piece of tissue to the other. Moreover, the rectum and distal sigmoid colon may be spared affecting only more proximal portions. Hence, it is imperative that endoscopists thoroughly sample all portions of the colon and perform generous sampling. Not uncommonly, one or two pieces of tissue are received in the lab with a request to rule out microscopic colitis, and while the limited sample may appear normal under the microscope, the limited sampling in and of itself inevitably puts the interpretation at risk for a clinically false negative. Care should be taken to consider differential diagnoses such as lymphocytic colitis, radiation colitis, ischemic colitis, mucosal prolapse, inflammatory bowel disease, amyloidosis, and normal mucosa with a thick basement membrane. While some of these may show deposition of pink amorphous materials, hyaline, or fibrin, the location and quality of the deposits should steer one in the right direction. Although generally not needed, for challenging cases ancillary stains such as trichrome and Congo red can be very helpful.
On rare occasions, CC may present with pseudomembranes aptly termed pseudomembranous collagenous colitis. Pseudomembranous colitis is a pattern of injury in which inflamed colonic tissue is covered by dense layers of fibrinopurulent exudates which endoscopically produces grey membranes and is generally associated with Clostridium difficile, E. Coli, ischemia producing drugs (i.e. NSAIDs) and ischemia (8). In pseudomembranous collagenous colitis, there is a similar injury pattern which happens secondary to collagen deposition. Endoscopically, along with membranes, red spots, vascular prominence, or linear mucosal defects can be seen. By histology, ulcerations, marked acute inflammation, crypt abscesses, and membranous exudates are seen in addition to an increased subepithelial collagen table. The diagnosis depends both on the recognition of the subtle background changes of CC as well as the clinical exclusion of other common causes of pseudomembranes. The treatment of this variant is like classical CC: anti-inflammatory agents such as budesonide (9,10).
References
1. Lindström CG. ‘Collagenous colitis’ with watery diarrhoea—a new entity? Pathol Eur. 1976;11(1):87–89.
2. Schein, J. Syndrome of non-tropical sprue with hitherto undescribed lesion of the intestine. Gastroenterology 1947. 8 (4):438–460.
3. Weinstein, W. M. , D. R. Saunders , G. N. Tytgat , and
C. E. Rubin . Collagenous sprue—an unrecognized type of malabsorption. N Engl J Med 1970. 283 (24): 1297–1301.
4. Xiangrong Zhao and Rebecca L. Johnson (2011) Collagenous Sprue: A Rare, Severe Small-Bowel Malabsorptive Disorder. Archives of Pathology & Laboratory Medicine: June 2011, Vol. 135, No. 6,
pp. 803-809.
5. Tong J, Zheng Q, Zhang C, Lo R, Shen J, Ran
Z. Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:265–276.
6. Boland K, Nguyen GC. Microscopic Colitis: A Review of Collagenous and Lymphocytic Colitis. Gastroenterol Hepatol (NY). 2017;13(11):671–677.
7. Gentile N, Yen EF. Prevalence, Pathogenesis, Diagnosis, and Management of Microscopic Colitis. Gut Liver. 2018;12(3):227–235.
8. Yuan, Shan MD; Reyes, Victoria MD; Bronner, Mary
P. MD Pseudomembranous Collagenous Colitis, The American Journal of Surgical Pathology: October 2003 -Volume 27 – Issue 10 – p 1375-1379.
9. Grunwald D, Mehta M, Sheth SG. Pseudomembranous Collagenous Colitis: A Case of Not-so-Microscopic Colitis. ACG Case Rep J. 2016;3(4):e187.
10. O’Toole A. Optimal management of collagenous colitis: a review. Clin Exp Gastroenterol. 2016;9:31–39.
