By Reenu Malhotra, M.D.
Malakoplakia, derived from the Greek adjective malakos (soft) and plaka (plaque), was first described in 1902 by Michaelis and Gutmann (1). It occurs predominantly in the genitourinary tract accounting for about 75% of the reported cases (2). The second most common site is the gastrointestinal tract, and most of the cases involve rectum and colon. It has also been found in many other sites such as the pancreas, liver, lymph nodes, retroperitoneum, skin, respiratory tract, adrenal gland, vagina and brain (1). An increase in association between malakoplakia and immunosuppression has been reported in literature (3).
 Malakoplakia in the colon has been described either as part of a disease with a diffuse presentation such as ulcerative colitis, diverticular disease, or as a rare incidental finding associated with an adenocarci-noma. Endoscopically, it can present with diffuse mucosal involvement and submucosal thickening and may produce a mass like appearance. The association with adenomatous polyps is very rare and till date only few reports have described such an association (3).
Malakoplakia in the colon has been described either as part of a disease with a diffuse presentation such as ulcerative colitis, diverticular disease, or as a rare incidental finding associated with an adenocarci-noma. Endoscopically, it can present with diffuse mucosal involvement and submucosal thickening and may produce a mass like appearance. The association with adenomatous polyps is very rare and till date only few reports have described such an association (3).
The association of malakoplakia and colonic adenocarcinoma is rare, with most cases described in the literature occurring in the rectum and sigmoid colon with a male predominance of 4:1. Only one case has been reported of similar association in the descending colon. In a series of four patients, malakoplakia associated with colorectal cancer tends to occur in elderly males in the rectum. The malakoplakia is found at the infiltrating front of the tumor and is not admixed with the neoplastic glands (4,5,6).
Definitive diagnosis of Malakoplakia can be made by histologic examination, the lesion is characterized by aggregates of histiocytes with abundant eosinophilic cytoplasm (Hansemann cells), admixed with lymphocytes, plasma cells and neutrophils. The diagnostic finding is presence of Michaelis-Gutmann bodies, 2-10 μm in size, with targetoid appearance due to concentric laminations, which stain with periodic acid-Schiff and von Kossa calcium stains. These bodies are phagolysomes, which are encrusted with calcium and iron salts and at ultrastructural level, reveal disin-tegrated bacteria. The origin of Michaelis-Gutmann bodies is most likely an abnormal response resulting in incompletely digested bacterial fragments and subsequent mineralization (1).
Malakoplakia is related to chronic bacterial infections, such as Escherichia coli, Proteus mirabilis, Staphylococcus aureus, Mycobacterium Tuberculosis and Shigella boydii. In patients with AIDS, Rhodococcus equi has also been reported (7). The pathogenesis of malakoplakia remains unknown. Three possible pathogenetic mechanisms have been suggested: an unusual causative organism, an abnormal or altered immune response and an abnormal macrophage response due to defective lysosomal function (8).
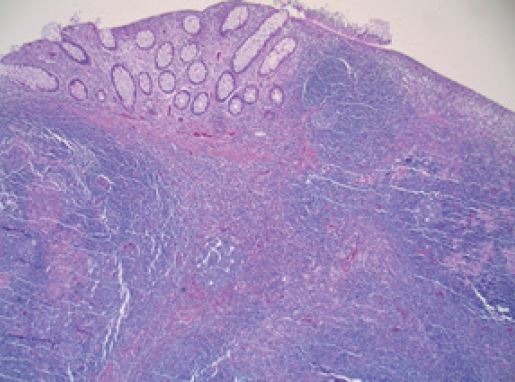
Fig.1: Reactive lymphoid hyperplasia associated with Malakoplakia
It is important to be aware of the existence of this entity in the gastrointestinal tract. In many situations, malignancy can be mimicked, especially when the lesion is polypoid, nodular, ulcerated and is accompanied by lymph node involvement. Pathologists should be also aware of the possibility of malakoplakia coexisting with other lesions, such as carcinoma in the same specimen. When the two conditions co-exist, malakoplakia is localized and almost always occurs adjacent to the invasive tumor, supporting the hypoth-esis of an unusual stromal response to the carcinoma. The presence of malakoplakia in a colon biopsy should warrant a thorough evaluation of the patient for a coexisting adenocarcinoma. Apart from the possibility of diagnostic error, malakoplakia itself does not appear to alter the prognosis of the tumor (3,4,5).
We had six patients with a histologic diagnosis of colonic malakoplakia in our practice in the last seven years. The patients raged from 51 to 81 years with M;F ratio of 1:2. These patients presented with variable symptoms ranging from iron deficiency anemia, hematochezia and history of colon polyps. Two patients visited the office for screening. Malakoplakia was histologically diagnosed on tissue biopsies (Fig. 1 & 2) from ascending colon (3pts.) cecum (2pts.), rectum (2pts.), descending (1pts.) and sigmoid colon (1pt.). Two of the patients had associated adenomatous polyps, one with hyperplastic polyp and two with reactive lymphoid hyperplasia (Fig. 1). Two of the cases showed no other associated pathology. Three of the patients showed malakoplakia at two different colonic biopsy sites. In these biopsies, histologic diagnosis was confirmed with special stains and immunostains including Iron, Von kossa (Calcium stain), PAS and CD68 (histiocyte marker). In a recent study involving analysis of 26 cases of gastrointestinal malakoplakia, 20 of these were diagnosed on biopsy material (17- colon/Rectum, 2 stomach, 1 appendix). The rectum was the most frequent site followed by sigmoid colon, cecum, ascending and transverse colon. Majority of the patients came in for screening and 14 showed reactive lymphoid hyperplasia on histology suggesting inflammatory nature of this process (8).
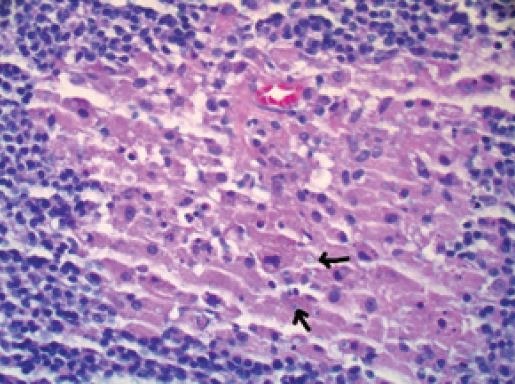
Fig.2: Histiocytes with targetoid Michaelis-Gutmann bodies
Although majority of reported cases of colonic malakoplakia have shown an association between systemic diseases and colorectal adenocarcinoma. There are some reported cases in literature which show colonic malakoplakia in association with adenomas (9, 10). Isolated cases of colonic malakoplakia have been reported without any association with systemic disease, adenocarcinoma or adenoma. These can present as isolated flat, soft and pale lesions on colonoscopic examination (11). The observation in our practice shows the rare coexistence of colonic malakoplakia with adenomatous polyps or presenting as an isolated polyp on colonoscopy. This confirms the incidental association of colonic malakoplakia with these entities and highlights the rarity of this association.
References
1. Long JP Jr, Althausen AF. Malacoplakia: a 25-year experience with a review of the literature. J Urol. 1989; 141:1328–1331.
2. Streem SB: Genitourinary malacoplakia in renal transplant recipients: pathogenic, prognostic and therapeutic considerations. J Urol. 1984 Jul; 132(1):10-2.
3. Pillay K, Chetty R. Malakoplakia in association with colorectal carcinoma: a series of four cases. Pathology 2002 Aug;34(4):332-5.
4. Asiyanbola B, Camuto P, Mansourian V. Malakoplakia occurring in association with colon adenocarcinoma.
J Gastrointest Surg. 2006; 10:657–661.
5. Bates AW, Dev S, Baithun SI. Malakoplakia and colorectal carcinoma. Postgrad Med J. 1997; 73:171–173
6. Schwartz DA, Ogden PO, Blumberg HM, Honig E. Pulmonary malakoplakia in a patient with the acquired immunodeficiency syndrome. Differential diagnostic considerations. Arch Pathol Lab Med. 1990; 114: 1267–1272
7. Lewin KJ, Fair WR, Steigbigel RT, Winberg CD, Droller MJ. Clinical and laboratory studies into the pathogenesis of malacoplakia. J Clin Pathol. 1976; 29:354–363
8. Yang Zhang, Kathleen Byrnes, MD et al: Gastrointestinal Malakoplakia Clinicopathologic Analysis of 26 Cases. Am J Surg Pathol. 2020 Apr 14. doi: 10.1097/
PAS.0000000000001491. [Epub)
9. Zanelli M , Ragazzi M2, Serra S1, Bellafiore S1, Ascani S3, De Marco L1. Malakoplakia Associated with Multiple Adenomas of the Colon: An Extremely Rare Incidental Finding. Int J Surg Pathol. 2016 Sep; 24(6):548-51.
10. Rizzo E, Sandmeier D, Hack I, Matter M, Bouzourene
H. Malakoplakia and colonic adenoma: a rare association. Ann Diagn Pathol. 2004; 8:364–366.
11. Yared RA,Badran HA et al. Colonic Malakoplakia: A Rare Finding in a Healthy Male. Case Rep Gastroenterol. 2018 Aug 21;12(2):453-456.
