By Cory A. Roberts, M.D.
This case is that of a 35 year old Asian American male. He presented with a long standing history of diarrhea (15 years). Subsequent endoscopic examination of his colon demonstrated areas of ulceration and grossly abnormal appearing mucosa in his cecum. The terminal ileum and much of the remaining colon appeared endoscopically normal. The rectum showed erythema but no ulceration. Each of these areas was biopsied with clinical concerns of Crohn’s disease and tuberculosis. The biopsies showed a focal active colitis with surface ulceration in the cecum. More importantly, numerous organisms occupied the surface of the colonic mucosa and were free floating in separate, detached fragments of
fibrin and mucus. In areas, one could see the organisms “erupting” from the areas of surface ulceration (figures 1 and 2). The organisms were large, round to oval and often featured a nucleus at one end. More prominent, however, was the presence of ingested fragmented erythrocytes. Each of these histological parameters led to the diagnosis of Entamoeba histolytica infection. Interestingly, none of the other biopsies away from the cecum showed organisms.
Entamoeba histolytica is protozoan which is prevalent in much of the world and even endemic in some developing countries. In the United States it is becoming more common in homosexual males but is otherwise uncommon in citizens of this country. Clinically, the history and appearance frequently lead the gastroenterologist to the brink of the diagnosis of Crohn’s before and after endoscopy. If the organisms cooperate, the pathologist can raise the idea of an amebic infection if they are present in great enough numbers to recognize and identify.1
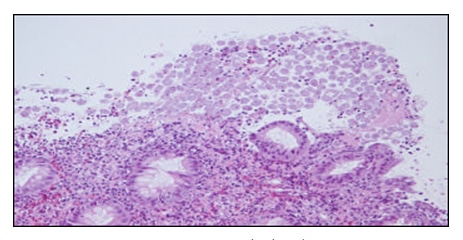
Figure 1: Entamoeba Histolytica
The amoebae enter the body as cysts that have been ingested inadvertently by the host by eating food or drinking water containing unknown fecal contamination. 2 The cysts are unscathed by the harsh environment of the gastric milieu. The trophozoites (eight per cyst) exit the cyst in the small bowel with assistance of chyme, proteases and pancreatic enzymes.3 The trophozoites inhabit the large bowel – most commonly the right side including a particular affinity for the cecum. The amoeba then must attach to the surface mucosal lining and live in harmony and with the support of already resident gut bacteria. Entamoeba histolytica then invades the bowel wall destroying host tissue with the aid of special enzymes and cytotoxins that it produces. These actions result in the tissue damage that produces the ulcers and erosions seen endoscopically – the socalled “flask-shaped ulcers”.1 While the pathologic stages of infestation show an intuitive progression from a mild, nonspecific colitis to deep ulceration with tissue necrosis, the fertile ground for finding the organisms remains the surface exudate regardless of the pathologic stage.2
The trophozoites may choose not to colonize the colonic mucosa. They may instead enter the portal circulation. If they do, they can then form amebic abscesses in the liver. The trophozoites themselves are not able to transmit the disease. While the cysts have no problem with gastric acid, trophozoites cannot survive that environment. Quadrinulceate cysts are formed from the trophozoites (encystation) which then allows the disease transmission.3 The liver abscess is the most frequent extraintestinal manifestation of disease and patients present with fever, right upper quadrant pain, hepatomegaly, weight loss, peripheral leukocytosis and elevated liver function tests, particularly alkaline phosphatase.4, 5
The clinical presentation of intestinal amebiasis is varied. Certainly, in the United States an appropriate travel history is always of great importance. Patients with E. histolytica present with diarrhea (sometimes bloody), abdominal pain, fever, tenesmus, weight loss and others may be relatively asymptomatic.2, 3
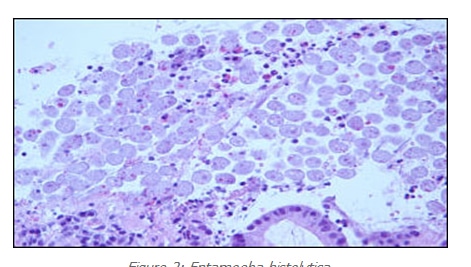
Figure 2: Entamoeba Hstolytica
Severe cases of amebiasis do exist. The organisms can produce a mass which may be mistaken for a tumor. These so-called “amebomas” can be a diagnostic challenge and may be relatively asymptomatic until obstructive symptoms occur. 6 These lesions can result in toxic megacolon and show necrosis of the bowel wall with perforations. The cecum is the most frequent site for ameboma and may be palpable on physical examination. The most dreaded presentation of intestinal amebic colitis is a fulminant necrotizing colitis that carries a 50% death rate. Necrotizing colitis most commonly occurs in children, pregnant women and/or patients on corticosteroids.5
The diagnosis can be made on clinical grounds and proven with biopsy. However, polymerase chain reaction (PCR) is sensitive, specific (can differentiate between pathogenic E. histolytica and nonpathogenic E. dispar) and now relatively easy. Serology (indirect hemagglutination assay) can detect the organism even in the case of hepatic abscesses but the patient can remain positive for years limiting the test’s diagnostic utility with patients from endemic regions of the world.7
Treatment with antibiotics is indicated and effective even in asymptomatic carriers from nonendemic regions of the world. Metronidazole is effective and inexpensive. Drainage of hepatic abscesses is not associated with improved recovery and is not indicated.8
A special thanks is in order to Dr. Son T. Do for providing this unique case.
References:
1. Ming S and Goldman H. Pathology of the gastrointestinal tract. 2nd edition. Williams and Wilkins. Baltimore 1998. pp 667
2. Scully R, Mark E, McNeely W, McNeely B. Case records of the Massachusetts general hospital case 18- 1990. N Engl J Med 1990 May 3;322(18):1298-305\
3. Katz D, Taylor D. Parasitic infections of the gastrointestinal tract. Gastroenterol Clin North Am 2001 Sep;30(3):797-815
4. Petri W. Recent advances in amebiasis. Crit Rev Clin Lab Sci 331:1-37, 1996.
5. Li E, Stanly SL. Protozoa: amebiasis. Gastroenterol Clin North Am 25:471-492. 1996.
6. Chandrasoma P. Gastrointestinal Pathology. Appleton and Lange. Stamford, CT. 1999. pp 262-3.
7. Kagan I. Serologic diagnosis of parasitic diseases. N Engl J Med 282:685, 1970.
8. van Allen R, Katz M, Johnson M. Uncomplicated amebic liver abscess: prospective evaluation of percutaneous therapeutic aspiration. Radiology 183:827, 1992.
