By ProPath Staff
Gastrointestinal stromal tumors (GISTs) are common mesenchymal tumors of the GI tract that are thought to arise from the interstitial cells of Cajal, normal cells in the wall of the gut that serves a pacemaker function. Studies have shown that >85% of GISTs have mutations involving the kit gene, which result in the constitutive activation of the kit receptor tyrosine kinase. Imatinib mesylate (a.k.a. STI 571, Gleevec, Glivec) is an inhibitor of the mutant c-kit kinase protein, and patients with GISTs have shown dramatic responses to therapy with this drug.
It has been well established that immunostaining with antibodies to c-kit (CD117) is very useful in the accurate diagnosis of GIST, and serves to identify patients who are likely to respond to imatinib. However, a small percentage of GISTs (probably in the range of 4-6%) do not demonstrate c-kit reactivity. Studies of these patients (with c-kit negative GISTs) have shown that a minority of them do harbor kit mutations, but this group is more likely to possess mutations of PDGFR-α, which, like the kit gene, codes for a protein with tyrosine kinase activity. Parenthetically, these c-kit negative GISTS may or may not express CD34. Importantly, some of these patients with c-kit negative GISTs that harbor PDGFR-α mutations (30% according to some studies) respond well to imatinib. There is some evidence to suggest that the kit mutations and PDGFR-αmutations are mutually exclusive.
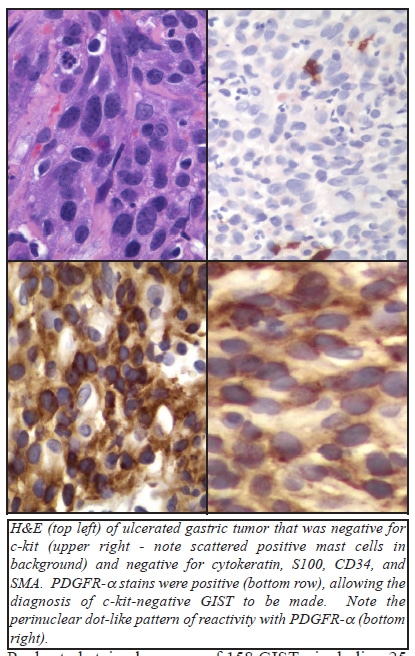
Until recently, the accurate identification of patients with c-kit negative GISTs has been problematic. However, several recent papers have reported on the utility of immunostains for PDGFR-α in identifying this group of patients.
Pauls et al stained a group of 158 GISTs, including 25 with PDGFR-α mutations, all of which occurred in the stomach. 92% of the PDGFR-α mutation group showed epithelioid or mixed epithelioid-spindle cell morphology, and all tumors with an epithelioid component had giant cells containing 2 or 3 tumor nuclei. All 25 of the PDGFR-α mutated tumors had moderate to strong PDGFR-α immunoreactivity. Strong c-kit reactivity was only found in 3 (12%) of these 25 PDGFR-α mutated cases, and moderate c-kit reactivity was found in 12 (48%) of these cases. Seven of these 25 (28%) showed weak reactivity, and 3 (12%) showed no immunoreactivity for c-kit. 98% of the kit-mutated GISTs (n=112) showed moderate to strong c-kit expression. Most of the c-kit mutated GISTs also stained for PDGFR-α, with strong staining in 20%, moderate staining in 44%, weak staining in 28%, and 8% negative. Among 21 wild-type GISTs (that lacked detectable c-kit or PDGFR-α mutations), 76% showed moderate to strong c-kit staining and 81% showed moderate to strong staining for PDGFR-α (with coexpression in 86% of cases). The authors noted that dot-like staining with c-kit was prominent in 71% of the c-kit mutated GISTs, but was less commonly seen in other cases. Similarly, dot-like staining for PDGFR-α was noted in 64% of the PDGFR-α mutated GISTs, but in only 13% of the c-kit mutated GISTs, and 33% of the wild-type GISTs.
Rossi et al evaluated the utility of immunostains for PDGFR-α in the differential diagnosis of c-kit negative GISTs and other mesenchymal tumors of the GI tract. They studied 125 GISTs, including 117 (94%) that stained for c-kit, and 8 cases (6%) that did not stain for c-kit. All of the c-kit positive GISTs lacked staining for PDGFR-α (in contrast to the results reported by Pauls et al), but all 8 of the c-kit negative GISTs showed immunoreactivity for PDGFR-α. Of the group of 42 non-GIST tumors studied (15 cases of extra-abdominal desmoid, 12 leiomyomas, 8 leiomyosarcomas, 3 schwannomas, 2 solitary fibrous tumors, 1 inflammatory pseudotumor, and 1 inflammatory fibroid polyp), only 4 of 15 (27%) of the desmoid tumors showed immunoreactivity for PDGFR-α. The authors did note that the PDGFR-α antibody was “troublesome”, at first, secondary to unwanted background staining, but that eventually, they were able to obtain a satisfactory signal-to-noise ratio. In their study, the authors defined “positive” as tumors that showed moderate to strong reactivity in >10% of the neoplastic cells. Some authors have noted that c-kit negative GISTs are more likely to show epithelioid morphology, occur in the stomach, omentum, or peritoneum, and may have multinucleated giant cells.
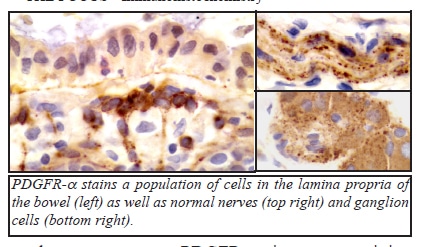
PDGFR-α stains normal ganglion cells and schwann cells in the bowel wall, serving as a convenient internal control in most cases. It also stains a subpopulation of cells in the lamina propria of the bowel. We have also seen PDGFR-α staining in neuroendocrine tumors, spermatogonia, thyroid follicular cells, and in a recent case of malignant fibrous histiocytoma.
We recently acquired the PDGFR-α antibody used in the studies cited below, and have been able to successfully identify several c-kit negative GISTs. To assist in interpretation, we perform the stain routinely at 2 different titers. PDGFR-α immunostains are now available at PROPATH for any interested clients.
References:
1. Medeiros F et al: KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol 28(7): 889- 894, July 2004.
2. Pauls K et al: PDGFRα- and c-kit mutated gastrointestinal stromal tumours (GISTs) are characterized by distinctive histological and immunohistochemical features. Histopathology 46: 166-175, 2005.
3. Rossi G et al: PDGFR expression in differential diagnosis between KIT-negative gastrointestinal stromal tumours and other primary soft tissue tumours of the gastrointestinal tract. Histopathology 46: 522-531, 2005.
4. Yi ES et al: Epithelioid gastrointestinal stromal tumor with PDGFRA activating mutation and immunoreactivity. Appl Immunohistochem Mol Morphol 13(2): 157-161, June 2005.
