By ProPath Staff
Many surgical pathologists are called upon to evaluate small intestinal biopsies from patients with diarrhea or other findings of malabsorption, and one of the entities that often enters into the differential diagnosis is gluten sensitivity (a.k.a. celiac disease, celiac sprue, nontropical sprue, or gluten-sensitive enteropathy). In a classic florid case, the small intestinal biopsy shows marked shortening or absence of the villi, and the diagnosis in this situation is readily made.
In a recent issue of the American Journal of Clinical Pathology, Goldstein and Underhill published an interesting study on the diagnosis of gluten sensitivity in architecturally normal duodenal biopsy specimens. They noted that a number of other investigators have found that up to 20- 50% of gluten-sensitive patients present with symptoms other than those associated with classic malabsorption, including microcytic anemia, folate deficiency, mild diarrhea, neurologic complaints, and severe osteoporosis. This has raised concerns that many patients with gluten sensitivity might remain undiagnosed. For this reason, some authors have recommended that the distal duodenum or jejunum be explored and biopsied in all patients undergoing upper GI endoscopy, so that more patients with gluten sensitivity may be recognized and appropriately treated.
In their paper, Goldstein and Underhill called attention to the fact that architecturally normal small bowel villi may be present in a biopsy from patients who are suffering from significant gluten sensitivity, and they sought to identify features that may assist in the identification of such patients. One of the features that they studied was the number of intraepithelial lymphocytes (IEL’s) at the tips of the villi, as well as the pattern and distribution of the villus IEL’s.
In normal patients, there were very few IEL’s in the villi, or there was a sparse IEL infiltrate in the basal portions of the villi that progressively disappeared as one followed the villi from the base to the tip (referred to as a “decrescendo” pattern). They found that an even distribution of the IEL’s along the villi (from the base up along the sides of the villi to the tips) was a sensitive marker of gluten sensitivity. The specificity of this change was limited however, as 25% of the patients with this finding did not have gluten sensitivity. In addition, increased IEL’s at the tips of the villi was suggestive but not diagnostic of gluten sensitivity. Therefore, pathologists who routinely examine duodenal or jejunal biopsy specimens should make a point to evaluate the presence and distribution of IEL’s in small intestinal villi, as they may be able to assist in recognizing patients with clinically unsuspected or clinically atypical gluten sensitivity. Other clinical situations that may be associated with increased IEL’s include food allergies, infections, common variable immunodeficiency, selective IgA deficiency, certain immune disorders (including Hashimoto thyroiditis, rheumatoid arthritis, etc.), and some patients taking NSAID’s. This finding was also recently described in obese patients undergoing gastric bypass surgery for the management of obesity.
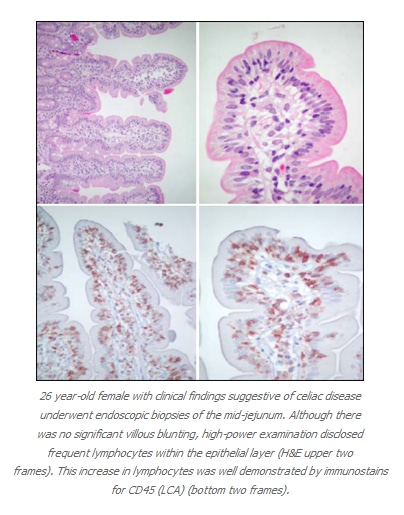
Although I do not mean to advocate that immunostains are required for evaluation of IEL’s, as an immunohistochemist I cannot resist the urge on occasion to perform some immunostains for my own enjoyment (at no charge to the patient) (see illustrations on the previous page). However, an article published in 2000 in Histopathology suggests that there can be a role for Immunohistochemistry in the evaluation of patients with refractory sprue, a condition in which patients do not respond to a gluten-free diet, with persistent malabsorption and small bowel mucosal abnormalities. The authors of this study found that typical gluten sensitivity is characterized by the presence of intraepithelial lymphocytes that are CD3 positive and CD8 positive (which is the same phenotype as the smaller numbers of intraepithelial T-lymphocytes found in control patients without gluten sensitivity). In contrast, the patients with refractory sprue contain CD3-positive intraepithelial lymphocytes that lack expression of CD8. Both of these antibodies are available for use with paraffin-embedded formalin-fixed material, so immunohistochemistry can serve a useful role in attempting to distinguish gluten sensitivity from refractory sprue on routine biopsy material.
REFERENCES:
1. Goldstein NS, Underhill J: Morphologic Features Suggestive of Gluten Sensitivity in Architecturally Normal Duodenal Biopsy Specimens. American Journal of Clinical Pathology 116: 63-71, 2001.
2. Moskaluk CA: Sailing Past the Horizon. The Histologic Diagnosis of Celiac Disease in “Non Flat” Intestinal Mucosa. (Editorial) American Journal of Clinical Pathology 116:7-9, 2001.
3. Robert ME, Ament ME, Weinstein WM: The Histologic Spectrum and Clinical Outcome of Refractory and Unclassified Sprue. American Journal of Surgical Pathology 24 (5):676-687, 2000.
4. Patey-Mariaud de Serre N, Cellier C, Jabri B et al: Distinction Between Coeliac Disease and Refractory Sprue: A Simple Immunohistochemical Method. Histopathology 37:70-77, 2000.
5. ElDeiry D, McKenna BJ: Intraepithelial Lymphocytosis in Patients Undergoing Gastric Bypass for Management of Obesity. Modern Pathology15 (1): 126A (abstract #523), 2002.
6. Kakar S, Nehra V, Murray JA et al: Intraepithelial Lymphocytosis in Small Bowel Biopsies with Normal Villus Architecture. Modern Pathology 15 (1): 131A (abstract #544), 2002.
